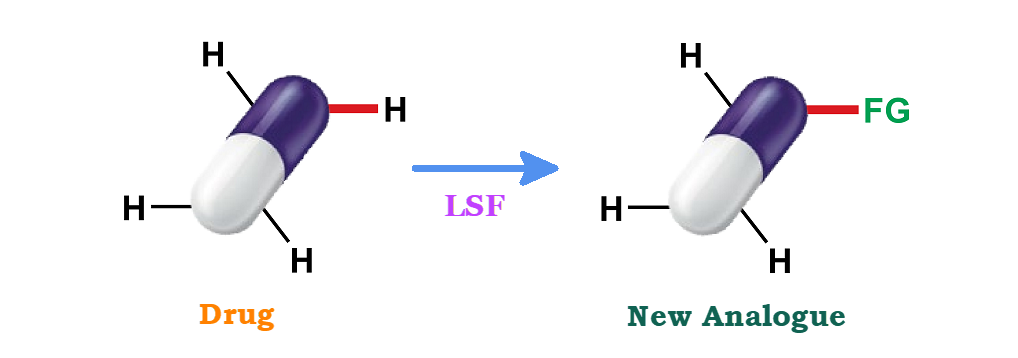
A minor functional modification to the structure of a drug candidate can have a profound impact on its biological profile. However, the optimization process in the final stage of drug development is often costly, complex, and time-consuming, especially when a complete de novo synthesis is required for structure activity relationship (SAR) based or artificial intelligence (AI)-aided target molecules. To address this issue, the recent concept “Late Stage Functionalization” (LSF) offers a potential solution. LSFs are highly versatile methodologies that effectively perform desired chemoselective transformations on intricate molecules to deliver numerous diverse products in a single reaction. LSF, especially when applied to the diversification of C-H bonds, presents a promising opportunity to explore new chemical space, particularly with sp3-carbon atoms. For late-stage diversification to become a valuable tool in drug discovery, advancements in high-throughput technologies and predictive techniques are necessary to accelerate the biological testing of the leads generated through this approach.
Drug discovery and development is a laborious, exceedingly costly, and highly precarious undertaking. The clinical progression of a drug candidate, spanning from the initiation of a clinical trial to obtaining marketing approval, demonstrates a discouragingly low rate of success (10%-20%) and necessitates a substantial investment, thereby demanding significant incentives for pioneering advancements in chemical synthesis to enhance production efficiency. Recently, the LSF reaction methodology has advanced to the point where it has started to have an impact in the field of medicinal chemistry. It can now be effectively utilized for reactions that require mild conditions and tolerance of various sensitive functional groups. In the past decade, there has been a significant increase in publications on various late-stage transformations, as evidenced by a comprehensive literature search conducted in Scopus, Web of Science, and SciFinder, as shown in Figure 1 [1]. The analysis of this literature search indicates that the present LSF toolbox encompasses numerous chemical and enzymatic methods, including fluorination, amination, hydroxylation, and methylation.
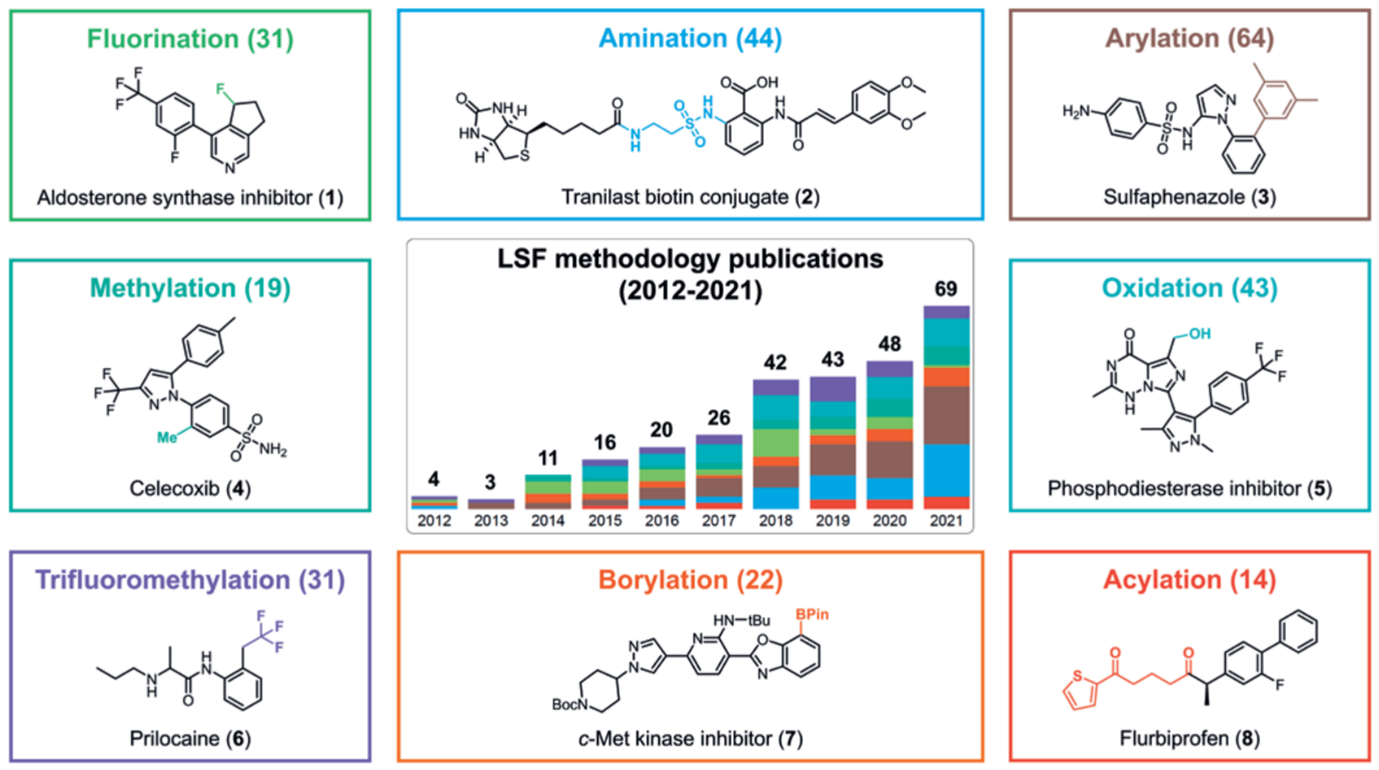
Figure 1: Increasing use of LSF.
LSF is a valuable tool for accessing derivatives that would otherwise be challenging or time-consuming to produce. Its utility extends to various applications, including SAR of pharmaceutical candidates and the creation of molecules with unstable isotopes that would not survive long enough for traditional synthesis methods. While LSF cannot replace de novo synthesis in all cases, it offers a means to access molecules that would have otherwise remained undiscovered. The ability to selectively diversify structurally complex molecules at a late stage holds significant potential for drug discovery. The desired features and useful tools of LSF have been demonstrated in Figure 2 [2]. In recent years, medicinal chemists have incorporated LSF strategies into their drug discovery programs, resulting in efficient access to diverse libraries for exploring structure-activity relationships and improving physicochemical and pharmacokinetic properties. Notably, substantial advancements have been made in providing reaction conditions for functionalizing sp2 or sp3 C-H bonds without the need for a synthetic handles.
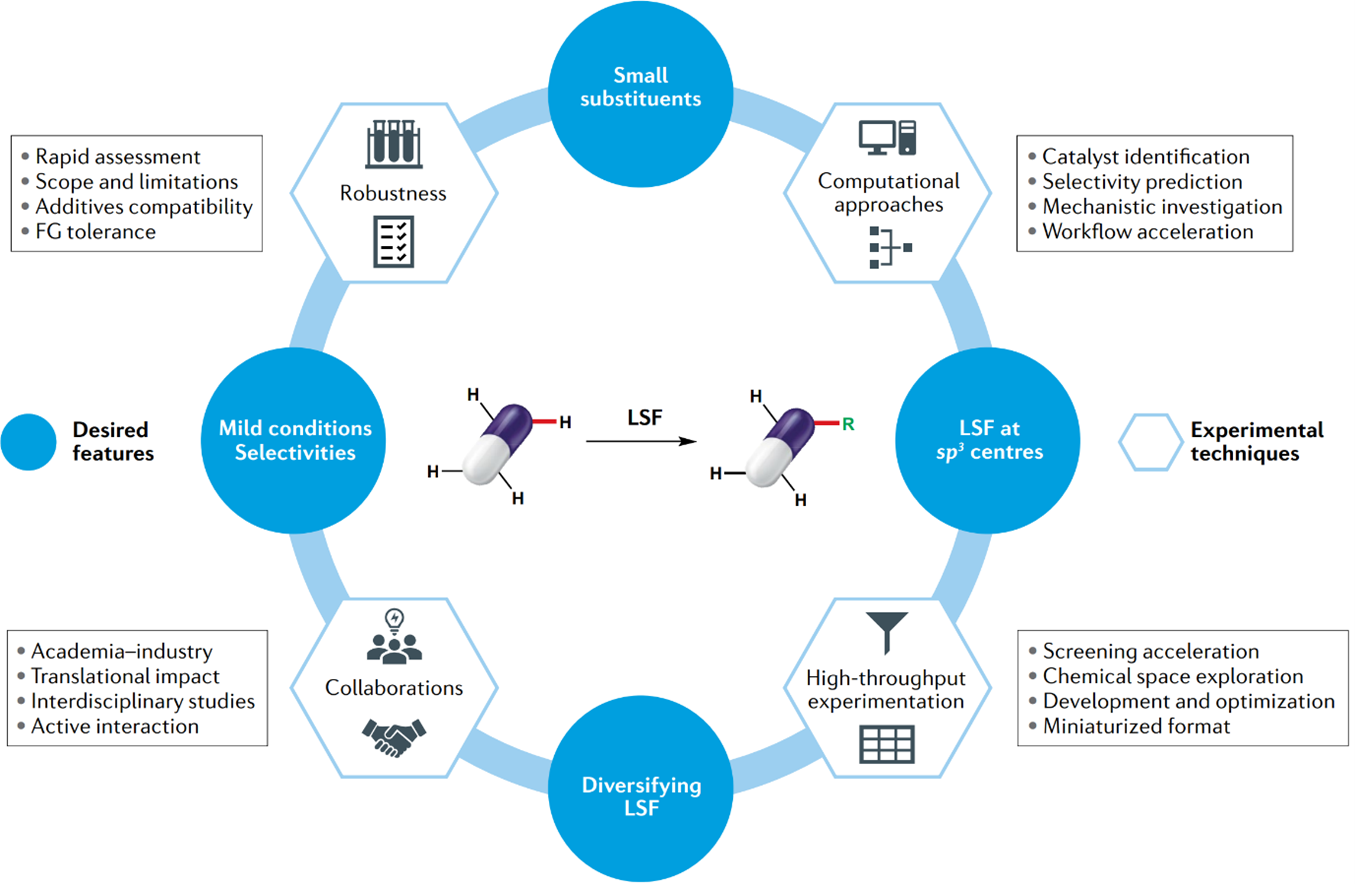
Figure 2: Desired LSF transformations and required features
The functionalization of C-H bonds offers significant opportunities for more efficient exploration of chemical space compared to traditional synthetic strategies, eliminating the need for extensive redesign of synthetic routes. Specifically, C-H bond late-stage functionalization enables the direct and selective conversion of prevalent C-H bonds into various C-X bonds (such as C-C, C-N, C-O, C-halogen) in complex molecular structures, without the requirement of pre-installing a functional group. This approach presents a straightforward and cost-effective method to accelerate the optimization of ADME profiles, physical properties, and the identification of structure-activity relationships, ultimately enhancing on-target potency and reducing off-target effects. Notably, the conversion of a C-H bond to C-CH3, known as the “Magic Methyl” effect (Figure 3) [3], exemplifies the profound pharmacological effects achievable. The ability to enable late-stage C-H functionalization in bioactive molecules holds significant value for drug discovery and development.
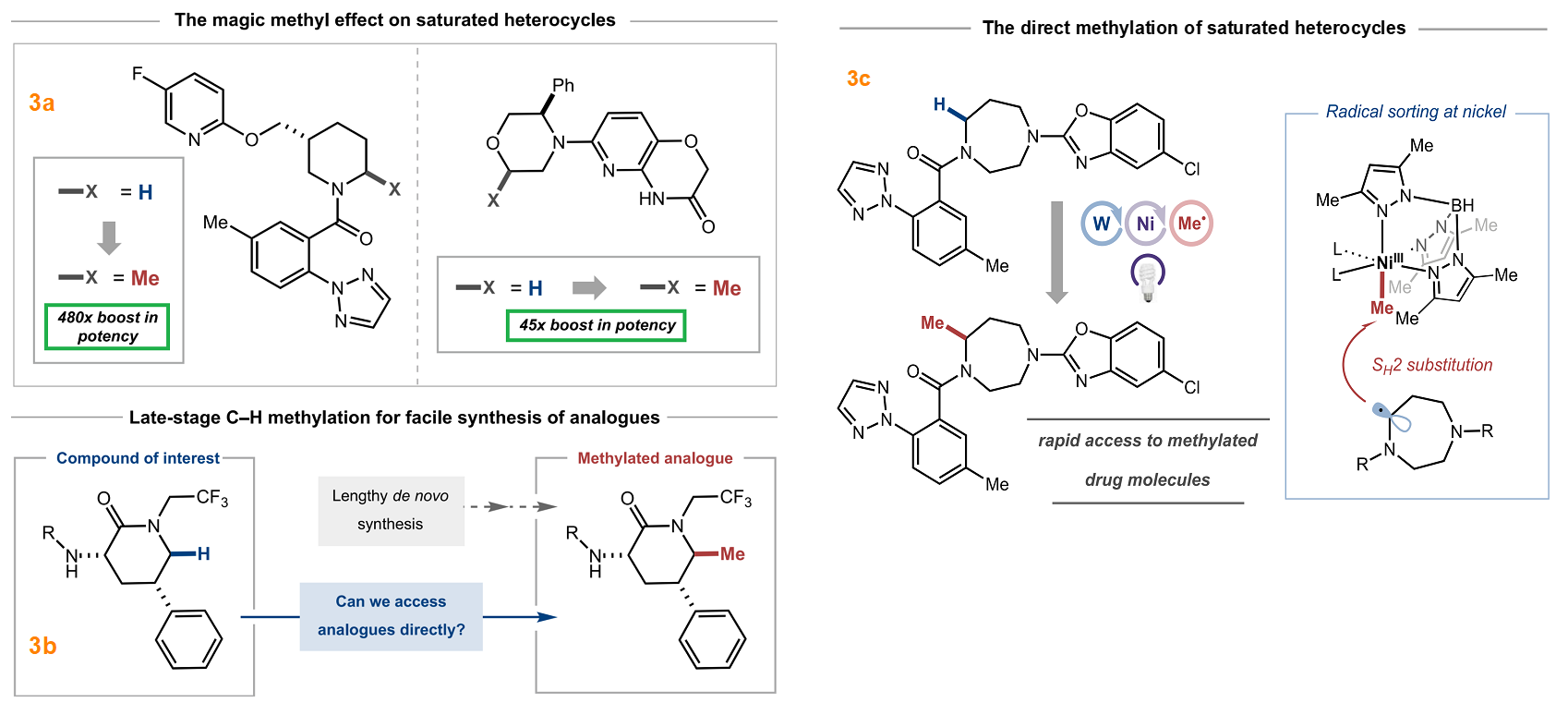
Figure 3: Magic Methyl Effect
Despite the significant progress made, strategies for LSF are still in the early stages of development. There are several challenges that need to be addressed in order to expand the range of methodologies applicable to pharmaceuticals. The functionalization of a drug candidate through C–H bonds may be limited due to selectivity issues that arise in complex molecules, or the lack of existing methodologies for the desired C–H functionalization. Most drug-like molecules have multiple reactive sites and their own complexities, resulting in a distinct reactivity pattern for each compound. Consequently, it is challenging to provide a comprehensive method that can overcome all the practical difficulties associated with LSF approaches. Currently, LSF of complex molecules is an immature approach with limited scope and success rates, often accompanied by inadequate control over chemoselectivity and/or regioselectivity.
References
[1] David F. Nippa, Remo Hohler, et al; Chimia 2022, 76, 258–260
[2] Lucas Guillemard, Nikolaos Kaplaneris, et al; Nat. Rev. Chem. 2021, 5, 522-545
[3] Edna Mao and David W.C. MacMillan; J. Am. Chem. Soc. 2023, 145, 2787−2793