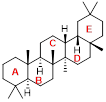
Oleanane as an example of a natural triterpenoid.
Triterpenes, such as oleanane, figure 1, are a class of chemical compounds with a carbon skeleton based on six isoprene units which are derived biossynthetically from the acyclic C30 hydrocarbon, squalene; they may also be thought of as consisting of three terpene units. Triterpenoids are functionalized triterpenes and are widely distributed in nature, they can be found in animals, plants and fungi and all produce triterpenes, including squalene, the precursor to all steroids.
Triterpenoids are very interesting from a chemical point of view since they have a variety of functional groups as well as from a pharmaceutical point of view since they possess a rich pharmacology (e.g. oleanolic acid, figure 1) with several pentacyclic motifs.
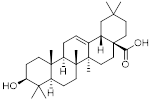
Oleanolic acid. It exhibits antitumor and antiviral properties.
Biosynthesis of triterpenoids in cells involves a handful of enzymes and can be divided into four stages. The formation of geranyl diphosphate from isopentenyl diphosphate units, formation of farnesyl diphosphate which is then converted to squalene and epoxysqualene. In the last stage, epoxysqualene is cyclized to the triterpenoid. This complicated pathway shows that it’s a challenging task for a synthetic chemist to synthesize triterpenoids in a laboratory setting.
The main synthetic strategies described in literature for pentacyclic triterpenes are:
(a) AB + DE ABCDE
(b) BC ABC ABC(E) ABCDE
(c) CDE BCDE ABCDE
(d) D(E) ABCD(E) via polyene cyclization ( ABCDE)
AAPharmaSyn has a lot of experience in using approach (b) in which a BC-ring system is first converted into an ABC system. Subsequent attachment of ring E and a consecutive cyclization finally affords the ABCDE ring system.
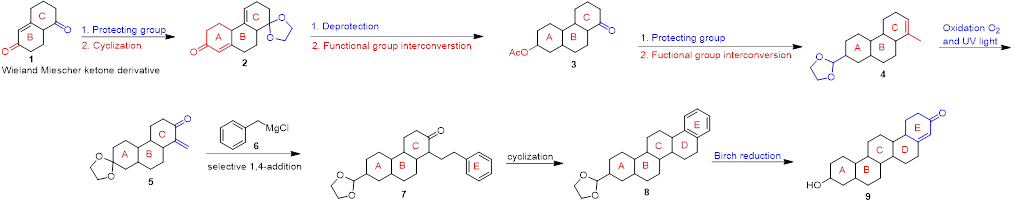
Scheme 1. Synthesis of a Triterpenoid 9.
An example of such a synthesis is shown in scheme 1 and starts with a Wieland Miescher ketone derivative. 1. Using commercially available or literature known building blocks can be very time saving and is a strategy that’s applied at AAPharmaSyn whenever possible. Functional group protection / interconversion / deprotection, techniques that are applied frequently at AAPharmaSyn, and in which we have ample experience, leads to intermediate 4. Oxidation of 4 with elemental oxygen followed by a selective 1,4-addition reaction of the Grignard-reagent 6, yields 7, the ABC(E)-system. Conversion of compound 5 to 7 is an example for a thoughtful and deliberately executed synthetic step, a strategy used at AAPharmaSyn in order to limit the amount of total steps needed and/or to improve yields. Cyclization of 7, followed by a Birch reduction finally affords compound 9, an ABCDE triterpenoid. Reactions such as the Birch reduction, the Benkeser reaction or the Bouveault-Blanc reduction are just a few examples of reactions performed at AAPharmaSyn.
![]()
Gly-Gly-Gly as an example of a Tripeptide. Peptide bond is shown in blue.
If the amine and carboxylic acid functional groups in amino acids join together to form amide bonds, a chain of amino acid units, called a peptide, is formed. A tripeptide (figure 1, example) is a peptide derived from three amino acids joined by two or sometimes three peptide bonds. A tripeptide composed of three different amino acids can be made in 6 different constitutions. When all twenty of the natural amino acids are possible components of a peptide, the possible combinations are enormous. As for proteins, the function of peptides is determined by the consistuent amino acids and their sequence. The simplest tripeptide is Gly-Gly-Gly (glycylglycylglycine). The tripeptide Ala-Ala-Ala for example serves as an exquisite model of sheet-forming peptide because it can be easily prepared as either a P or an AP β-sheet structure.
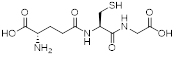
Tripeptide glutathione (γ-Glu-Cys-Gly).
In terms of scientific investigations, the simple and widely distributed tripeptide glutathione is interesting because the side-chain carboxyl function of the N-terminal glutamic acid is used for the peptide bond and it serves many roles in many forms of life. Glutathione is a tripeptide of glutamic acid, cysteine, and glycine with a peptide linkage between the γ-carboxyl group of the glutamate side-chain and the amine group of cysteine (figure 2). Glutathione exists not only in mammals but also in plants and fungi, proving this compound has particularly important roles in living creatures. In fact, glutathione is a powerful antioxidant whose efficacy is based on the thiol group of cysteine. It detoxifies hydrogen peroxide into water and reduces disulfide bonds formed within proteins by oxidative stress into cysteine.
Synthesis of Tripeptides
The synthesis of a specific dipeptide (or any peptide) such as Ala-Gly from alanine and glycine is complicated, because both amino acids have two functional groups. This can result in the formation of 4 different products: Ala-Ala, Ala-Gly, Gly-Ala, and Gly-Gly. Therefore, the synthesis of a specific peptide requires modification of both amino acids. One amino acid has to be protected at its carboxyl group, and the second amino acid is protected at the amino group. Then, only one condensation reaction is possible. AAPharmaSyn is very experienced in coupling reactions to form amide bonds using all sorts of coupling reagents such as HBTU, HATU, HOBt, CDI, DCC, EDAC, DIC, and DMAP. For successful peptide coupling reactions, it is imperative to have a broad knowledge of coupling reagents since none of the available coupling reagents is applicable to all types of coupling reactions.
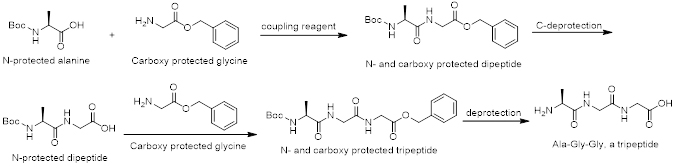
Scheme 1. A typical tripeptide synthesis, here Ala-Gly-Gly.
Scheme 1 shows the synthesis of a tripeptide (Ala-Gly-Gly) which can also be applied for the synthesis of other tripeptides. AAPharmaSyn has the necessary experience and know-how to synthesize tripeptides fast and efficiently.
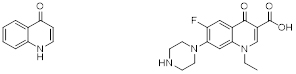
4-Quinolone (left), and Norfloxacin as an example of a synthetic quinolone (right).
Quinolones are a type of antibiotic. Antibiotics kill or inhibit the growth of bacteria. A quinolone antibiotic is a member of a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. There are five different quinolone classes but nearly all quinolone antibiotics in use are fluoroquinolones, which contain a fluorine atom in their chemical structure and are effective against both Gram-negative and Gram-positive bacteria. Examples are norfloxacin and ciprofloxacin, one of the most widely used antibiotics worldwide. Quinolones and fluoroquinolones have many things in common, but also a few differences such as what organisms they are effective against. One common thing for example is that both detrimentally affect the function of two enzymes produced by bacteria, topoisomerase IV and DNA gyrase, so that they can no longer repair DNA or help in its manufacture. However, because of their risk of serious side effects, the FDA has advised that they are not suitable for common conditions such as sinusitis, bronchitis, and uncomplicated urinary tract infections, and should only be considered when treatment with other, less toxic antibiotics, has failed. Quinolones and fluoroquinolones may also be used to treat unusual infections such as anthrax or plague. Doctors may also decide to use them for other types of infection when other alternative treatment options have failed or cannot be used.
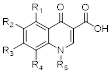
Core structure of quinolone antibiotics. There are 5 important positions for modifications to improve the activity of the drug: R1, R2, R3, R4, R5.
Synthesis of Quinolones
The synthesis of quinolones often involves ring-closing reactions. Such reactions often install a carbonyl group (4-quinolones) across from the ring nitrogen. Examples are shown in scheme 1.
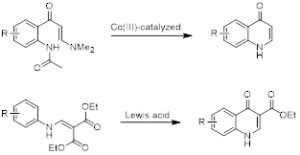
Scheme 1. Ring-closing reactions for the synthesis of 4-Quinolones.
Depending on the nature of the substituents R, displacement reactions can be performed in order to change the substitution pattern of the quinolone on the benzene ring. AAPharmaSyn has performed a vast number of such benzene ring manipulation reactions as well as displacement reactions and was able to gain a lot of experience and knowledge on how to strategically approach the synthesis of various substituted quinolones.
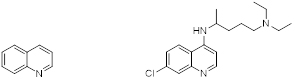
Quinoline (left), and Chloroquine as an example of a synthetic quinoline (right).
Quinoline (figure 1, left) is a heterocyclic aromatic organic compound with the chemical formula C9H7N. Quinoline itself has only a few applications, but many of its derivatives are useful in diverse applications such as antimalarial agents. A prominent example is chloroquine (figure 1, right) which is used for the prophylaxis and treatment of malaria attacks by for example Plasmodium vivax and also against chloroquine-sensitive strains of Plasmodium falciparum.
Quinine, a naturally occurring quinoline alkaloide has been used as an oral medicine for the prevention of malaria for almost 400 years. Quinine and its stereoisomer quinidine when administered intravenously can be used against acute malarial infections. During an intense program of research to eradicate malaria in the mid 20th century, thousands of compounds were synthesized and tested for efficacy in animal models. Pamaquine, an 8-amino substituted quinoline derivative, was identified as a potent antimalarial agent
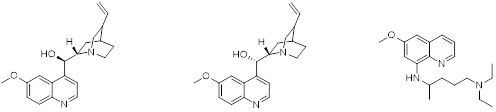
Quinine (left), Quinidine (middle), Pamaquine (right)
Just recently, Washington University School of Medicine in St. Louis was launching a clinical trial for patients hospitalized with COVID-19 at Barnes-Jewish Hospital. The trial will investigate the effectiveness of different combinations of the antimalarial drugs chloroquine and hydroxychloroquine and the antibiotic azithromycin in treating ill patients infected with the novel coronavirus. The Food and Drug Administration recently gave emergency approval for hospitals across the country to use the two antimalarial drugs to treat severe cases of COVID-19. However, this treatment strategy remains unproven.
Synthesis of Quinolones
Quinolines can be synthesized in multiple ways. Examples are the Combes quinoline synthesis, Conrad-Limpach reaction, Doebner reaction and the Skraup synthesis (scheme 1). Quinoline is an important heterocyclic derivative that serves as a building block for many pharmacological synthetic compounds.
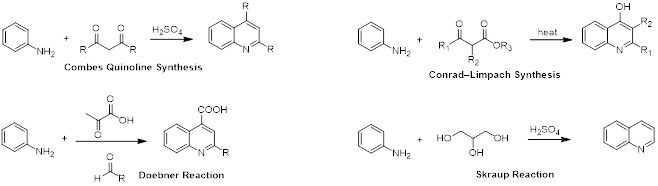
Scheme 1. Synthesis of substituted and unsubstituted Quinolines.
AAPharmaSyn has ample experience in synthesizing and derivatizing quinolines either using synthetic strategies shown in scheme 1 or in-house developed synthetic methods. We also use quinolines as building blocks to access and synthesize more complex and synthetically challenging compounds.
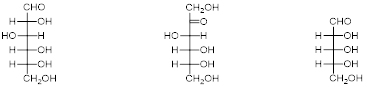
D-Glucose (left), D-Fructose (middle), and D-Ribose (right).
A carbohydrate is a molecule consisting of carbon, hydrogen, and oxygen atoms and are polyhydroxy aldehydes or ketones or are compounds that can be broken down to form such compounds. Usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula Cx(H2O)y (where x may be different from y). This formula applies to most monosaccharides but isn’t true for deoxyribose, a sugar component of DNA which has the empirical formula C5H10O4. The most common carbohydrate is glucose (C6H12O6), an aldohexose, figure 1, left. Other examples include Fructose (C6H12O6), a ketohexose, figure 1, middle and Ribose (C5H10O5), an aldopentose, figure 1, right.
A glucuronide, also known as glucuronoside, is a molecule that can be obtained by linking glucuronic acid to another substance via a glycosidic bond. The conversion of chemical compounds to glucuronides, is an ability animals have to assist in the excretion of drugs or other (toxic) substances that cannot be used as an energy source. Glucuronides possess a much higher water solubility than the original substance, and therefore can eventually be excreted by the kidneys.
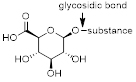
Example of a glucuronide; glucuronic acid is attached to a substance via a glycosidic bond.
Carbohydrates are considered as the next frontier in pharmaceutical research. Synthetic carbohydrates are used to study their roles in biological important processes such as cell-cell recognition, immunological response, metastasis, and inflammation. Successful applications of carbohydrates as active agents are for example, fully synthetic oligosaccharide vaccines to combat tropical diseases (e.g., malaria), bacterial infections (e.g., tuberculosis), viral infections such as HIV, and cancer. Carbohydrates are also used extensively in the pharmaceutical sector as excipients to facilitate packaging and delivery of drugs. These formats include tablets, capsules, liquids, suspensions, gels, inhalation products and strips.
Due to the multi-functional nature of carbohydrates they can undergo various chemical conversions with a variety of reagents. Some examples of D-Glucose conversions are shown in figure.
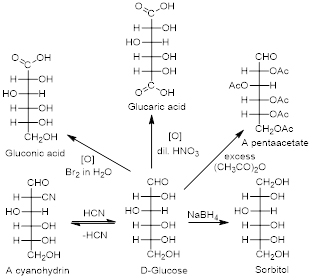
Glucose and other carbohydrates can react with a variety of reagents.
AAPharmaSyn has synthesized many glucuronides and has done multiple functional group interconversions on glucose and other aldohexoses and ketohexoses. Carbohydrate chemistry can be tricky at times and requires careful and deligent planning before starting the synthesis; a strenghth that AAPharmaSyn has and which is highly valued by our customers.
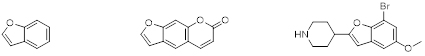
Benzofuran left, Psoralen, middle, as an example of a naturally occurring substituted benzofuran, and Brofaromine, right, as an example of a synthetic substituted benzofuran.
Benzofuran is one of the most important fused heterocycles, containing a benzene ring fused to a furan ring (figure 1). The substituted benzofurans are the scaffold of many natural products and several complex structures. Benzofurans have attracted widespread attention due to their biological activity and they are also present in numerous naturally occurring products. Many pharmaceutically active compounds derived from benzofuran have a benzofuran core to which a 2-aminoethyl group is attached (at any position), and combined with a range of other substituents. An example of a naturally occurring substituted benzofuran is psoralen (figure 1, middle) which can be found in large number of plants such as celery, parsley, and in all citrus fruits. An example for a synthetic substituted benzofuran is brofaromine (figure 1, right), it is a reversible inhibitor of monoamine oxidase and was primarily researched in the treatment of depression and anxiety.
Synthesis of Substituted Benzofurans
Due to their remarkable activity as agrochemicals and pharmaceuticals, many synthetic approaches to substituted benzofurans have been explored and described in literature. Those approaches include acid-catalyzed cyclizations of carbonyl compounds via dehydration, ring closure using an intramolecular Wittig reaction, generation of ketene as intermediate followed by cyclization, Dieckmann condensation of activated methylene, intramolecular Friedel–Crafts reaction, and also a modified Fisher indole synthesis. However, one of the best methods for an access to benzofuran derivatives is via a Sonogashira cross-coupling reaction between o-halo phenol and terminal alkynes followed by sequential 5-endo-dig cyclization, scheme 1.
![]()
Synthesis of 2-substituted benzofurans using Sonogashira reaction conditions.
In the last decade or two, palladium cross-coupling reactions have gained more and more attention and those reactions were not only applied in total syntheses of natural products but for all kinds of products. This is also reflected in the fact that in 2010, Heck, Negishi, and Suzuki were awarded the Nobel Prize in Chemistry for the development of palladium catalyzed cross-coupling reactions.
For many years, AAPharmaSyn has utilized those cross-coupling reactions for the purpose of reducing the total amount of steps towards target molecules and to increase the total overall yield which saves costs and time. AAPharmaSyn not only has ample experience with palladium catalyzed cross coupling reactions such as Suzuki, Sonogashira, Heck, Negishi, Hiyama, Buchwald-Hartwig, and Stille but also with non palladium based catalyzed reactions such as the Chan-Lam or Ullmann coupling reaction.
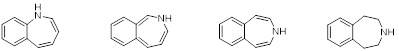
From left to right: 1-Benzazepine, 2-Benzazepine, 3-Benzazepine and Tetrahydro-3-benzazepine.
Benzazepines are heterocyclic chemical compounds with a benzene ring fused to a seven membered aza-heterocyclic (azepine) ring, figure 1. Azepine rings are constituents of a number of compounds with interesting and diversified pharmacological properties. The tetrahydro-3-benzazepine ring system is of particular interest from a medicinal chemistry point of view because it contains the phenethylamine nucleus, an essential part of several neurotransmitters and corresponding drugs, embedded in its structure.
The Benzazepine moiety is attracting a lot of attention due to its wide spectrum of biological activity mostly on central nervous system (CNS). The synthesis of the benzazepine scaffold has been investigated and reported by a number of researchers and its presence is noticed in nature as a basic structure in some alkaloids. Not limited to CNS, benzazepine derivatives are involved in a multitude of biological activities. Some examples are shown in figure 2. Therefore, development of new and efficient synthetic routes for these compounds is of high interest in heterocyclic chemistry. Hence, a number of research articles devoted to the chemistry, stereochemistry and biological activity of benzazepine and its derivatives are available in literature.

Left, Lorcaserin, a weight-loss drug, right, Fenoldopam an antihypertensive agent.
Synthesis of Benzazepines
A very common synthetic strategies for the formation of benzazepines are ring closure reactions onto an aryl ring which include for example a Heck reaction of an allyl amine with an aryl halide (scheme 1, right) or an intramolecular Friedel–Crafts cyclization reaction (scheme 1, left).

Synthesis of tetrahydro-3-benzazepine ring system via Friedel-Crafts-cyclization (left) or via Heck reaction (right).
AAPharmaSyn has synthesized many benzazepine ring system using either the Friedel-Crafts or Heck coupling reaction route. Our team was able to gather a great deal of knowledge and experience which helps in improving and optimizing the reaction conditions for better yields and shorter reaction times. Different substitution patters on the aromatic ring can make the ring closure reaction very challenging, especially when there are deactivating groups such as –NO2-substituents or substituents which direct the incoming group into the “wrong” position. With our knowledge and expertise we have overcome a lot of those challenges in most recent years.
![]()
Biguanide (left), Metformin an asymmetric dimethylbiguanide (middle), Buformin a butyl derivative of biguanide (right).
Biguanide (figure 1, left) is an organic colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to ammonia and urea. A variety of derivatives of biguanide are used as pharmaceutical drugs. Metformin (figure 1, middle) for example, an antidiabetics drug, is widely used in the treatment of diabetes mellitus type 2. There are several classes of blood glucose lowering medications and each class has its own mechanism of action. Metformin works by reducing glucose release from the liver, increasing the transport of glucose into the muscle, thereby increasing insulin sensitivity, and also decreasing the absorption of glucose from the gastrointestinal tract. Some biguanides are also used as antimalarial drugs, examples are Proguanil and Chlorproguanil (figure 2), both biguanide derivatives

Proguanil left and Chlorproguanil right.
Synthesis of Biguanide
Biguanide and its derivatives are synthetically accessible by reacting cyanoguanidine with ammoniumchloride or other substituted amines.
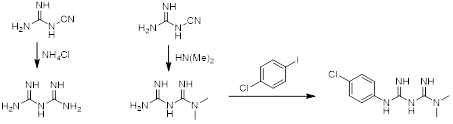
Scheme 1. General synthesis of biguanide (left) and dimethylbiguanide (middle) to Proguanil (right).
Our team at AAPharmaSyn not only has successfully synthesized many biguanides but also utilized them as the five-atom fragment for the synthesis of 1,3,5-triazines (scheme 2) which are useful building blocks for a variety of biologically active compounds. 2,4,6-Trichloro-1,3,5-triazine (cyanuric chloride) for example is the starting point for the manufacture of many herbicides such as Simazine and Atrazine.
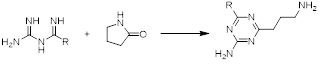
Scheme 2. Synthesis of substituted 1,3,5-triazine rings using biguanides.
AAPharmasyn has extensive experience in conducting multi-step syntheses of various classes of compounds. Our goal and focus is always to create the most value for our client and we frequently engage in custom synthesis of commercially available intermediates or building blocks if they are very expensive in order to reduce cost for the client. More often than not, we need to design a large scale synthetic procedure.
Scheme 1 shows an example of a multi-step synthesis which has been performed in a similar way by our experienced and talented team at AAPharmaSyn. In general, before we start a synthesis we engage in literature research in order to design the best possible retro synthesis for the target molecule. Even though, we can strongly rely on our experience and knowledge, we always use a scientific search to learn what is known about a given reaction and how that knowledge can be used in order to get the best yield possible and to reduce the formation of side products. In cases of completely unknown reactions, we approach the problem from a scientific point of view and try to weigh out in advance the pros and cons of using certain reaction conditions. As an example, the retrosynthetic analysis of compound 23 shows that a retro intramolecular aldol/dehydration step furnishes compound 22. Intramolecular aldol reactions are well known reactions and give very high yields. Retrosynthetic analysis of compound 21 led us to the conclusion that the tertiary allylic carbocation of 19 would participate in a polyolefinic cyclization reaction to yield 21 from 18. The retrosynthetic analysis of 15 going to 14 is another example of a strategic approach since the Wittig reaction is a well known literature procedure and compound 15 can easily be obtained from the reaction of 14 with 7 in high yields and trans selectively. These are just a couple of examples that illustrate how diligently synthetic routes are developed before reactions are carried out in our laboratory.
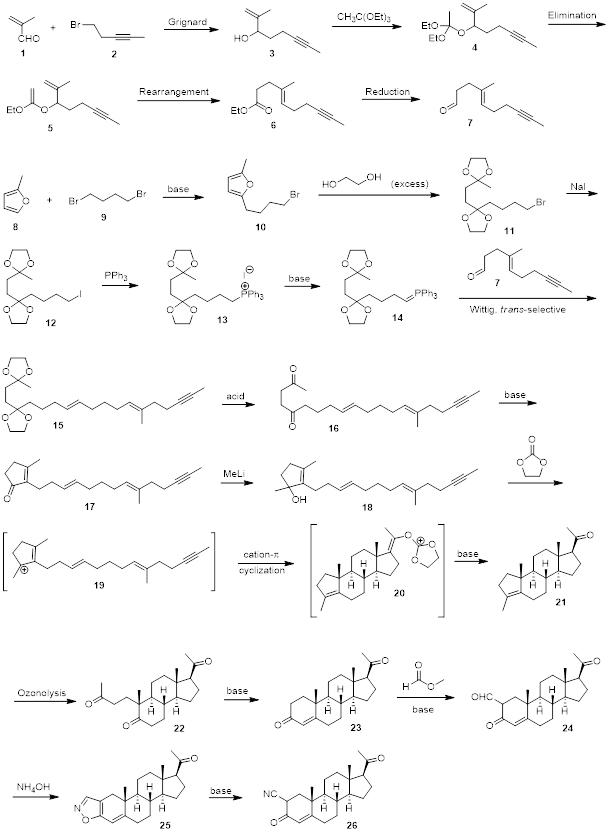
Scheme 1. Multi-step synthesis of a triterpenoid.
2019
Preparation of pyrazole and imidazole compounds for inhibition of IL-17 and RORγ
Jiang, Xin; Visnick, Melean; Bender, Christopher F.; Bolton, Gary; Caprathe, Bradley; Lee, Chitase; Kornberg, Brian; O’Brien, Patrick; Hotema, Martha R.
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2019241796 A1 (2019-12-19).
Biaryl amides with modified sugar groups for treatment of diseases associated with heat shock protein pathway
Jiang, Xin; Visnick, Melean; Bender, Christopher F.; Bolton, Gary; Caprathe, Bradley; Lee, Chitase.
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2019222269 A1 (2019-11-21).
2018
Preparation of pyrimidine tricyclic enone derivatives as RORγ inhibitors for the treatment and prevention of inflammation or autoimmune disorders
Jiang, Xin; Bender, Christopher F.; Visnick, Melean; Hotema, Martha R.; Sheldon, Zachary S.; Lee, Chitase; Caprathe, Bradley William; Bolton, Gary; Kornberg, Brian.
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2018111315 A1 (2018-06-21).
2017
Preparation of C4-modified oleanolic acid derivatives for inhibition of IL-17 and other uses
Visnick, Melean; Jiang, Xin; Hotema, Martha R.; Lee, Chitase; Caprathe, Bradley William; Roark, William H.; Bolton, Gary.
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2017053868 A1 (2017-03-30).
2016
Preparation of imidazolyl tricyclic enones as antioxidant inflammation modulators
Jiang, Xin; Caprathe, Bradley William; Lee, Chitase; Bolton, Gary; Bender, Christopher F.; Visnick, Melean.
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2016130927 A1 (2016-08-18).
2015
Preparation of aryl and arylalkyl substituted pyrazolyl and pyrimidinyl tricyclic enones as antioxidant inflammation modulators
Anderson, Eric; Jiang, Xin; Bender, Christopher; Bolton, Gary; Caprathe, Bradley William; Lee, Chitase; Roark, William; Donner, Pamela; Wagner, Rolf; Shanley, Jason; Heyman, Howard; Krueger, Allan; Chen, Hui-Ju; Rozema, Michael; Grampovnik, David; Visnick, Melean
(AbbVie Inc., USA; Reata Pharmaceuticals, Inc.). US Patent WO 2015112792 A1 (2015-07-30).
2012
Pyrazolyl and pyrimidinyl tricyclic enones as antioxidant inflammation modulators
Anderson, Eric; Bolton, Gary L.; Caprathe, Bradley; Jiang, Xin; Lee, Chitase; Roark, William H.; Visnick, Melean
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2012083306 A2 (2012-06-21).
2009
Preparation of natural product compounds for treatment of inflammatory diseases
Anderson, Eric; Bolton, Gary L.; Ferguson, Deborah A.; Jiang, Xin; Kral, Robert M., Jr.; O’Brian, Patrick M.; Visnick, Melean
(Reata Pharmaceuticals, Inc., USA). US Patent WO 2009146218 A2 (2009-12-03).


