Description
This compounds has uses in synthesis of potential drug candidates and as a negative control in biochemicals assays.
$470.00 – $4,310.00
This compounds has uses in synthesis of potential drug candidates and as a negative control in biochemicals assays.
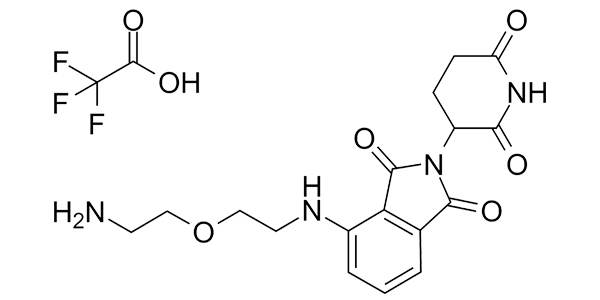
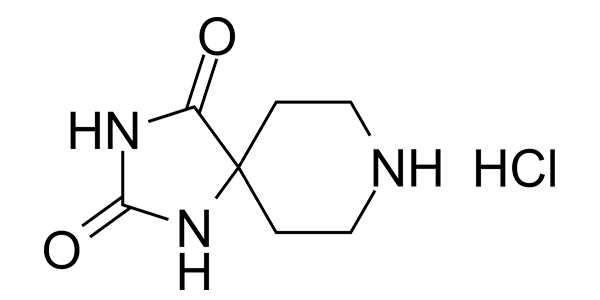
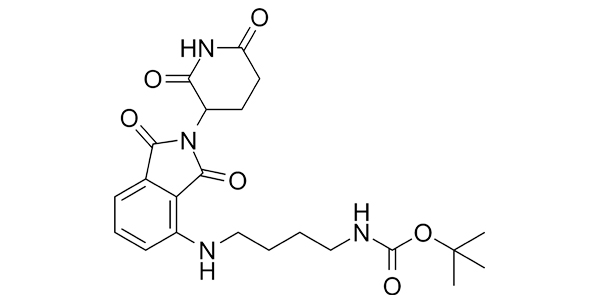
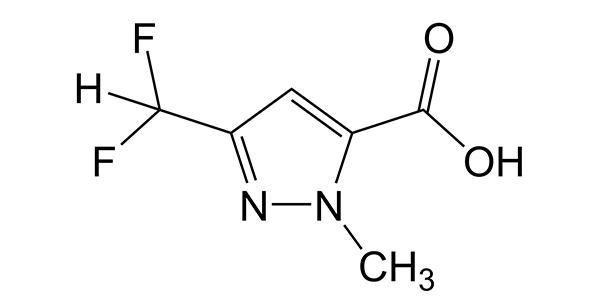
| Property Name | Property Value | Reference |
|---|---|---|
| Molecular Weight | 440.9 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Hydrogen Bond Donor Count | 4 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Hydrogen Bond Acceptor Count | 8 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Rotatable Bond Count | 10 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Exact Mass | 440.1462622 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Monoisotopic Mass | 440.1462622 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Topological Polar Surface Area | 140 Ų | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Heavy Atom Count | 30 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 642 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
| Undefined Atom Stereocenter Count | 1 | Computed by PubChem |
| Defined Bond Stereocenter Count | 0 | Computed by PubChem |
| Undefined Bond Stereocenter Count | 0 | Computed by PubChem |
| Covalently-Bonded Unit Count | 2 | Computed by PubChem |
| Compound Is Canonicalized | Yes | Computed by PubChem (release 2016.09.28) |
Room temperature free S&H from Ann Arbor, MI. Shipping on ice packs available upon request.
Storage stability not tested. Recommended storage at -20°C.
≥ 1 year
The present invention is related to the preparation of a bifunctional compound ULM-Linker-PTM [ULM = CRBN, VHL or IAP E3 ubiquitin ligase-binding moiety; PTM = HMG-CoA reductase binding moiety, especially a statin or a derivative thereof; Linker = a chem. group that links ULM and PTM], e.g., I, and a method for degradation of HMG-CoA reductase using them, and to their use for prevention or treatment of HMG-CoA reductase related diseases such as cardiovascular diseases and hyperlipidemia. Thus, I was prepared using (2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-[4-(4-methylthiazol-5-yl )benzyl]pyrrolidine-2-carboxamide, 1-(9H-fluoren-9-yl)-3-oxo-2,7,10,13,16,19-hexaoxa-4-azahenicosan-21-oic acid and 5-[[(1S,3R,7S,8S,8aR)-8-[2-((2R,4R)-4-hydroxy-6-oxotetrahydropyran-2-yl)ethyl]-3, 7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl]oxy]-4,4-dimethyl-5-oxopentanoic acid (preparation given). I was evaluated for its HMG-CoA reductase degradation-inducing activity in hepatocytes (DC50 < 50 nM).