Description
This compounds has uses in synthesis of potential drug candidates and as a negative control in biochemicals assays.
$350.00 – $2,850.00
This compounds has uses in synthesis of potential drug candidates and as a negative control in biochemicals assays.
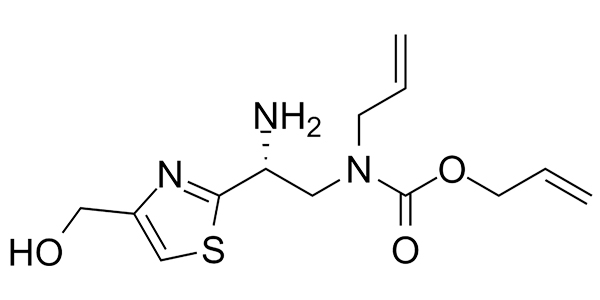
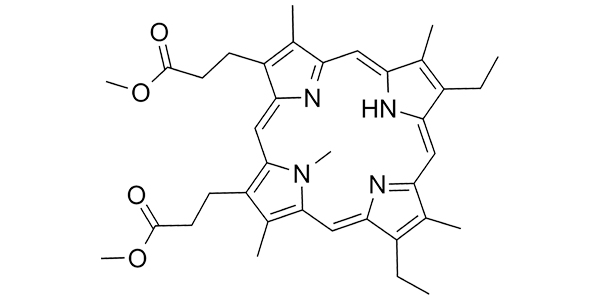
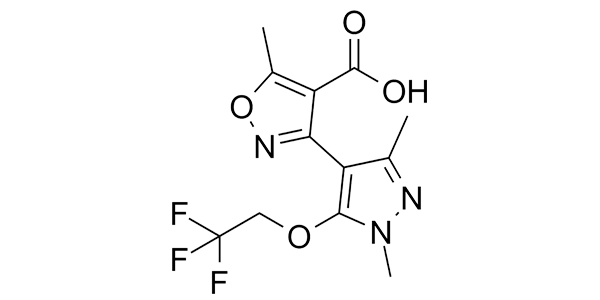
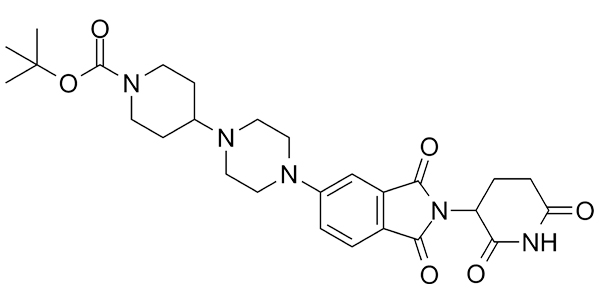
| Property Name | Property Value | Reference |
|---|---|---|
| Molecular Weight | 378.8 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Hydrogen Bond Donor Count | 3 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Hydrogen Bond Acceptor Count | 6 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Rotatable Bond Count | 2 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Exact Mass | 378.1094828 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Monoisotopic Mass | 378.1094828 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Topological Polar Surface Area | 98.8 Ų | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Heavy Atom Count | 26 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 616 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
| Undefined Atom Stereocenter Count | 1 | Computed by PubChem |
| Defined Bond Stereocenter Count | 0 | Computed by PubChem |
| Undefined Bond Stereocenter Count | 0 | Computed by PubChem |
| Covalently-Bonded Unit Count | 2 | Computed by PubChem |
| Compound Is Canonicalized | Yes | Computed by PubChem (release 2016.09.28) |
Room temperature free S&H from Ann Arbor, MI. Shipping on ice packs available upon request.
Storage stability not tested. Recommended storage at -20°C.
≥ 1 year
By: Min, Jaeki; Jarusiewicz, Jamie; Actis, Marisa; Chang, Yunchao; Alcock, Lisa; Mulligan, Charles; Rankovic, Zoran
World Intellectual Property Organization, WO2022133285 A1 2022-06-23 | Language: English, Database: CAplus
The present disclosure relates to pyrazolylpyrrolopyrimidine derivative chem. compounds I (q = 0 or 1; A = O, S, N H, etc.; L = C
alkyl, -(CH CH O) -, -(CH CH O) (C alkyl)-; Q = -C(O)(C H )-, -NHC(O)(C H )- and -C(O)NH(C H )-; R = C alkyl, hydroxyalkyl,
cyanoalkyl, etc.) that modulate JAK-2 signaling, which are useful in the treatment of disorders associated with J AK-2 signaling dysfun
ction such as, for example, disorders of cellular proliferation (e.g., cancer) and autoimmune and inflammatory disorders, pharmac
eutical compositions containing such compounds, and their use in treatment. This abstract is intended as a scanning tool for
purposes of searching in the particular art and is not intended to be limiting of the present invention.
By: Crew, Andrew P.; Hornberger, Keith R.; Wang, Jing; Dong, Hanqing; Qian, Yimin; Crews, Craig M.; Jaime-Figueroa, Saul
United States, US10723717 B2 2020-07-28 | Language: English, Database: CAplus
The disclosure relates to bifunctional compounds of formula I, which find utility as modulators of rapidly accelerated fibrosarcoma
(RAF, such as c-RAF, A-RAF and/or B-RAF, the target protein). In particular, the present disclosure is directed to bifunc tional
compounds I, wherein ULM is a small mol. E3 ubiquitin ligand binding moiety that binds to E3 ubiquitin ligase selected from a group
consisting of Von Hippel-Lindau, inhibitors of apoptosis proteins or mouse double- minute homolog 2 ligand; PTM is a small mol.
that binds the target protein RAF; L is a bond and a chem. linking moiety; and their pharmace utically acceptable salts, enantiomers,
stereoisomers, solvates, polymorphs and prodrugs thereof, are claimed. Thus, compound II was prepared by a multistep procedure
(procedure given). The invention compounds were evaluated for their B- RAF degradation activity. From the assay, it was determined
that compound II exhibited DC value in the range of < 100 n M to > 50 nM and D value of > 70%.
By: Crew, Andrew P.; Hornberger, Keith R.; Wang, Jing; Crews, Craig M.; Jaime-Figueroa, Saul; Dong, Hanqing; Qian, Yimin;
Zimmermann, Kurt
United States, US20200129627 A1 2020-04-30 | Language: English, Database: CAplus
The present disclosure relates bifunctional compounds of formula U LM-L-PTM, their use in modulators of Rapidly Accele rated
Fibrosarcoma (RAF, such as c-RAF, A-RAF and/or B-RAF; the target protein) and treatment of diseases that result from aggreg ation
or accumulation of the target protein. and their prepar ation Compounds of formula ULM-L-PTM, wherein ULM is a small mol. E3
ubiquitin ligase binding moiety; PTM is a small mol. comprising a R AF protein targeting moiety; L is a bond or chem. linking moiety
connecting ULM and PTM; and pharmaceutically acceptable salts, enantiomers, stereoisomers, solvates, polymorph and prodrug
thereof, are claimed. Compound I was prepared using a multistep procedure (procedure given) . The present disclosure exhibits a
broad range of pharmacol. activities associated with degradation/inhibition of target protein (data given) .