Description
This compounds has uses in synthesis of potential drug candidates and as a negative control in biochemicals assays.
$130.00 – $700.00
This compounds has uses in synthesis of potential drug candidates and as a negative control in biochemicals assays.
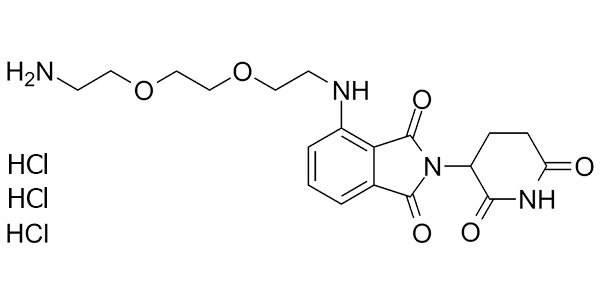
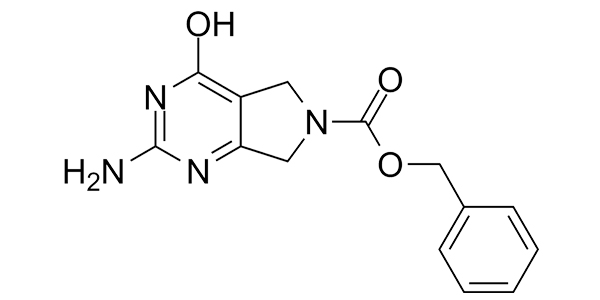
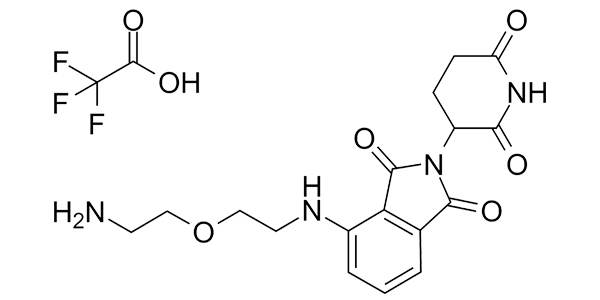
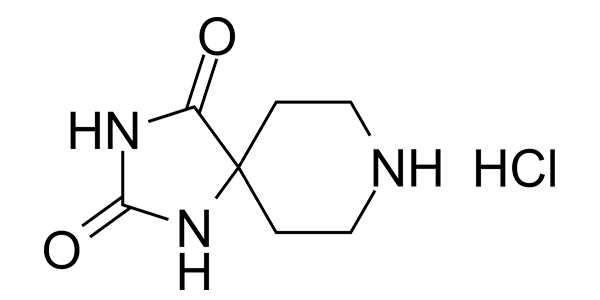
| Property Name | Property Value | Reference |
|---|---|---|
| Molecular Weight | 237.29 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| XLogP3-AA | 0.9 | Computed by XLogP3 3.0 (PubChem release 2021.05.07) |
| Hydrogen Bond Donor Count | 2 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Hydrogen Bond Acceptor Count | 3 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Rotatable Bond Count | 2 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Exact Mass | 237.13649347 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Monoisotopic Mass | 237.13649347 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
| Topological Polar Surface Area | 66.4 Ų | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Heavy Atom Count | 17 | Computed by PubChem |
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 376 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
| Undefined Atom Stereocenter Count | 2 | Computed by PubChem |
| Defined Bond Stereocenter Count | 0 | Computed by PubChem |
| Undefined Bond Stereocenter Count | 0 | Computed by PubChem |
| Covalently-Bonded Unit Count | 1 | Computed by PubChem |
| Compound Is Canonicalized | Yes | Computed by PubChem (release 2021.05.07) |
Room temperature free S&H from Ann Arbor, MI. Shipping on ice packs available upon request.
Storage stability not tested. Recommended storage at -20°C.
≥ 1 year
By: Molcanov, Kresimir; Kojic-Prodic, Biserka; Basaric, Nikola; Mlinaric-Majerski, Kata
Acta Crystallographica, Section E: Structure Reports Online (2006), 62(12), o5406-o5408 | Language: English, Database: CAplus
3-Acetamidoadamantane-1-carboxylic acid, C H NO , crystallizes with 2 mols., A and B, in the asym. unit. Intermol. O- H···O H
bonds link symmetry-related mols. into infinite chains parallel to the [101] direction, with short O···O distances of 2.534 (2) and 2.618
(2) Å in the chains of A and B mols., resp. Intermol. N- H···O H bonds cross-link these 2 chains. Crysta llog. data are given.
By: Novikov, S. S.; Khardin, A. P.; Butenko, L. N.; Kulev, I. A.; Novakov, I. A.; Radchenko, S. S.; Burdenko, S. S.
Zhurnal Organicheskoi Khimii (1980), 16(7), 1433-5 | Language: Russian, Database: CAplus
Reaction of I or II (R = H) with HNO -oleum at 10-15°, then with MeCN gave I or II (R = AcNH), hydrolysis of which with HCl gave
mixtures of I and II (R = NH , Cl); increasing the concent ration of HCl increased the yield of chloro compound
By: Xie, Bingyu; Guo, Jianwei; Liu, Sa; Peng, Jinping
Youji Huaxue (2011), 31(4), 486-489 | Language: Chinese, Database: CAplus
3-Amino-1-adamantanol, a key intermediate of anti-diabetic drug, was successfully synthesized via bromination, azido, Curtius
rearrangement and hydrolysis processes by using adamantane carboxylic acid as the starting raw material. The overall yield of 3-
amino-1-adamantanol was 34%. 3-Amino-1-adamantane carboxylic acid hydrochloride, a anti-neoplastic agents, was synthesized
from adamantane carboxylic acid by Ritter, hydrolysis reaction in total yield of 73%. The structures of products were identified by
elemental anal., IR, MS, H NMR, etc.