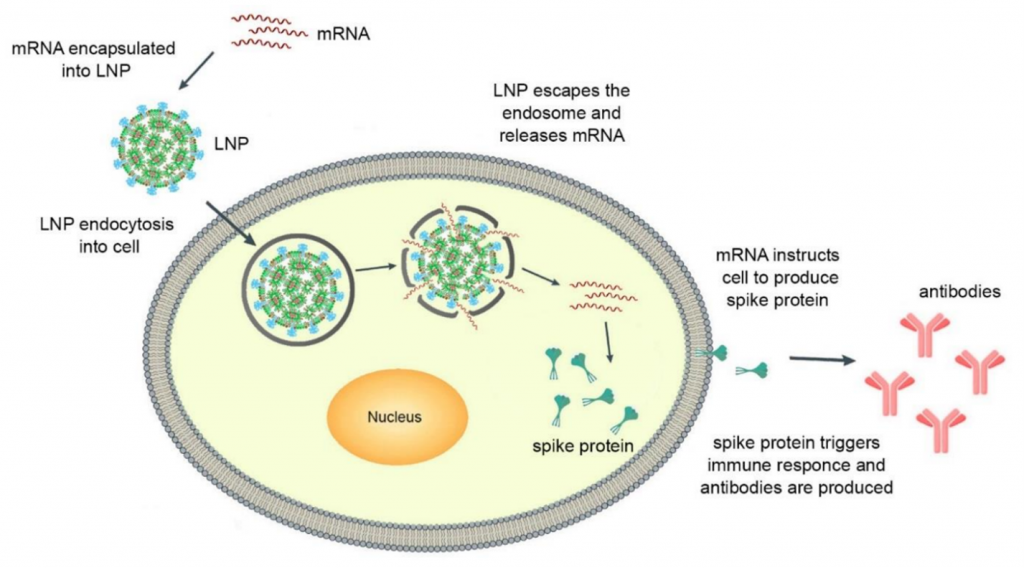
Nanomedicine, the field that encompasses the convergence of nanotechnology, pharmaceutical, and biomedical sciences, has experienced rapid advancements in recent years. Particularly notable is the development of new nanoformulations for therapeutic purposes, imaging agents, and theragnostic applications. One area of great promise within nanomedicine is the use of lipid-based nanoparticles for targeted delivery of nucleic acids to disease-causing active sites. These offer solutions to common challenges in drug delivery, such as low water solubility and poor bioavailability. Furthermore, lipid-based nanoparticles have the ability to overcome various physiological obstacles, enabling enhanced distribution to the desired sites. Recently, lipid nanoparticles (LNPs) have proven successful in efficiently delivering cytotoxic chemotherapy agents, antibiotics, and nucleic acid therapeutics. The significance of this field has been recognized by awarding the Nobel Prize of 2023 in Physiology or Medicine to Katalin Karikó and Drew Weissman for their discoveries enabling the creation of a new type of messenger RNA (mRNA) vaccine using LNPs as delivery vehicles. The mechanism of action of LNP-mRNA mediated vaccination is depicted in Figure 1 [1].
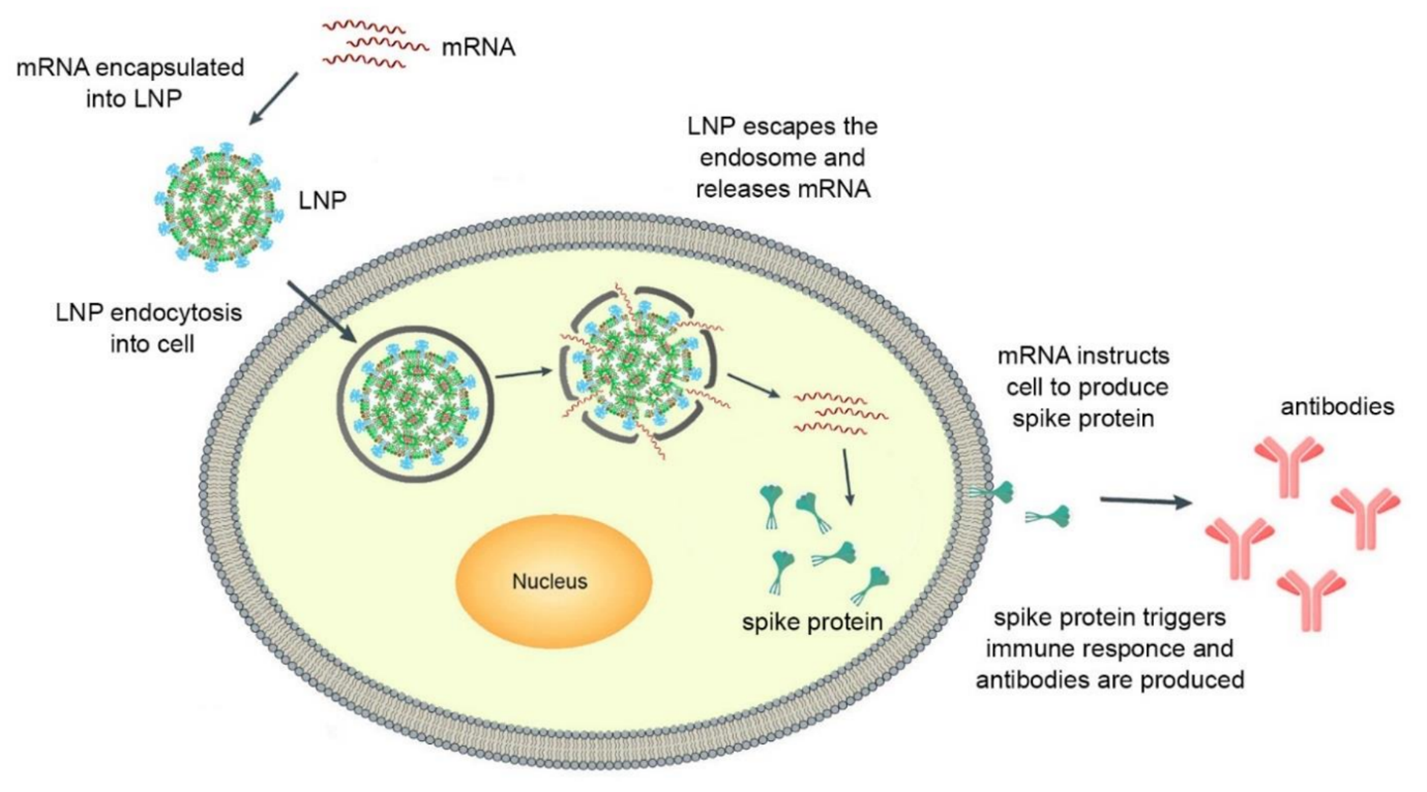
Figure 1. Mechanism of action of LNP-mRNA mediated vaccination.
RNA therapeutics show great potential in various medical applications, including virus vaccines, cancer immunotherapy, and gene editing. Various drug delivery vehicles, including LNPs, have been developed to address the instability of RNA. LNPs have been regarded as the most efficient and suitable delivery system for nucleic acids, such as small interfering RNA (siRNA) and mRNA. Liposomes, an early version of LNPs composed of phospholipids and cholesterol, were first used to deliver mRNA in 1978. Although liposomes have been explored for effective drug delivery for three decades, the first FDA approval came in the 1990s with Doxil®, a stealth liposome encapsulating doxorubicin. Doxil® has been clinically used to treat ovarian and metastatic breast cancer, as well as different forms of myeloma. Liposomes have continued to be successful in clinical applications, with over twenty liposomal products receiving FDA approval for encapsulating various small molecule drugs. The clinical success of Doxil® has paved the way for the FDA approval of many new nanodrugs, including Abelcet®, AmBisome®, DaunoXome®, Depocyt®, Inflexal V®, Myocet®, Visudyne®, DepoDur®, DepoCyt®, Marqibo®, Mepact®, Exparel®, Lipodox®, Onivyde®, Doxorubicin, Nocita®, Vyxeos®, Shingrix®, LipoplatinTM, and Arikayce® for a wide range of diseases [2].
To improve the encapsulation of charged mRNA, the development of cationic lipid-based LNPs and ionizable lipid-based LNPs has been undertaken. Currently, ionizable lipids are recognized as the essential components of LNP-based RNA therapeutics. They possess a positive charge at low pH to facilitate the encapsulation of negatively charged RNA. At physiological pH (~7.4), the charge of ionizable lipids becomes less positive or nearly neutral, thereby reducing toxicity. Moreover, various mRNA engineering techniques have been employed to improve the stability and translation efficacy of mRNA therapeutics. These methods include the selection of untranslated regions (UTRs), addition of a poly-A tail, capping, and nucleoside modification. Extensive global efforts are currently underway to advance LNP-based drug development, as illustrated in Figure 2 [1].
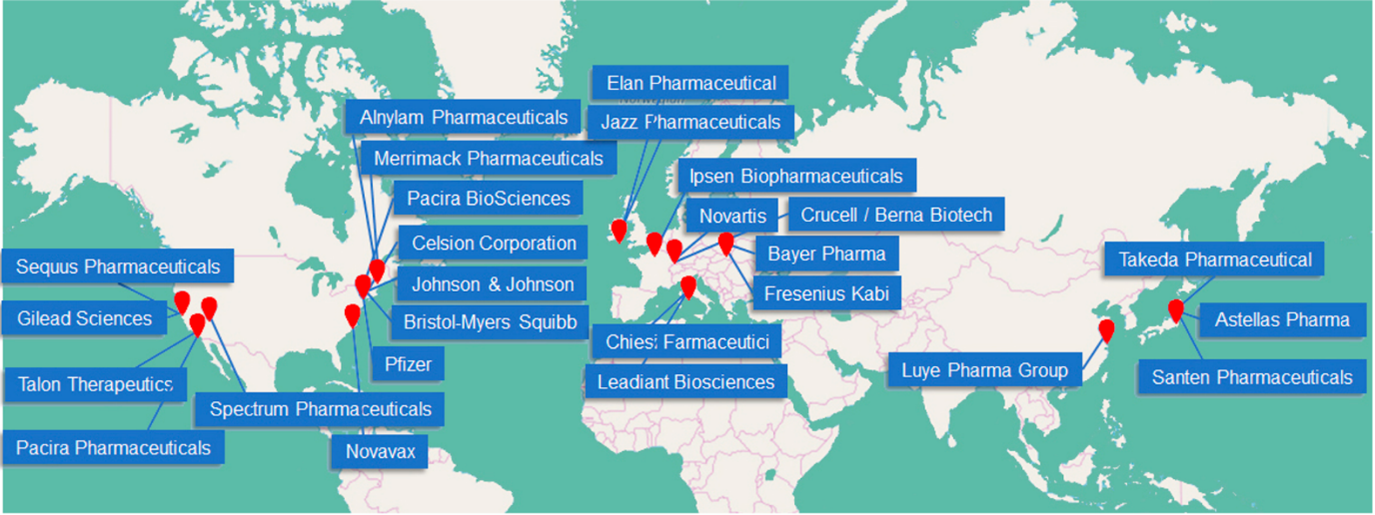
Figure 2. Key players operating in the global LNP drug delivery market according to a recent market analysis and the summary of the LNP-based marketed drugs.
The first LNP-formulated siRNA drug received FDA-approval in 2018 was Patisiran (ONPATTRO®) which was developed by Alnylam Pharmaceuticals, Inc. (Cambridge, MA, USA) and Sanofi Genzyme (Cambridge, MA, USA). This drug effectively reduces the production of transthyretin protein in the liver and is used for the treatment of hereditary transthyretin-mediated amyloidosis. Patisiran is composed of (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31-tetraen-19-yl-4-(dimethylamino)-butanoate (DLin-MC3-DMA) lipid, disterarolyphosphatidychloline (DSPC), cholesterol, and a PEG-lipid (PEG-DMG) that directs the particle in vivo towards liver hepatocytes. The amine head groups of the ionized lipid optimized for siRNA delivery play a crucial role in the efficacy of the drug. In 2019, another siRNA therapeutic, GIVLAARITM (Givosiran, ALN-AS1), received FDA approval. It targets the ALAS1 gene in hepatocytes and is prescribed for adult patients with the hereditary disease acute hepatic porphyria.
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection was declared a pandemic by the World Health Organization (WHO) in 2019. In response, two mRNA vaccines, namely Pfizer/BioNTech’s BNT162b2 and Moderna’s mRNA-1273, have been developed with unparalleled speed utilizing LNPs as delivery vehicle (Figure 3) [3]. These vaccines have demonstrated significant efficacy in preventing the disease. By delivering mRNA encoding for the SARS-CoV-2 spike protein into the cytoplasm of host cells, the vaccines stimulate the production of the spike protein which acts as an antigen, triggering the development of an immune response to the virus. These vaccines were granted emergency use authorization (EUA) by the FDA in December 2020 for COVID-19. The LNPs compositions of both mRNA vaccines exhibit remarkable similarities. They both consist of an ionizable lipid that carries a positive charge at low pH facilitating RNA complexation, and remains neutral at physiological pH. Additionally, a PEGylated lipid is incorporated to minimize antibody binding by serum proteins and clearance by phagocytes, thereby prolonging systemic circulation. Besides, the inclusion of phospholipid DSPC (distearoylphosphatidylcholine) and cholesterol assists in compacting the cargo within the LNPs. The molar ratios of the cationic lipid:PEG-lipid:cholesterol:DSPC are (46.3:1.6:42.7:9.4) for the Pfizer vaccine and (50:1.5:38.5:10) for the Moderna vaccine. These nanoparticles exhibit a diameter of 80−100 nm and contain approximately 100 mRNA molecules per LNP [1].
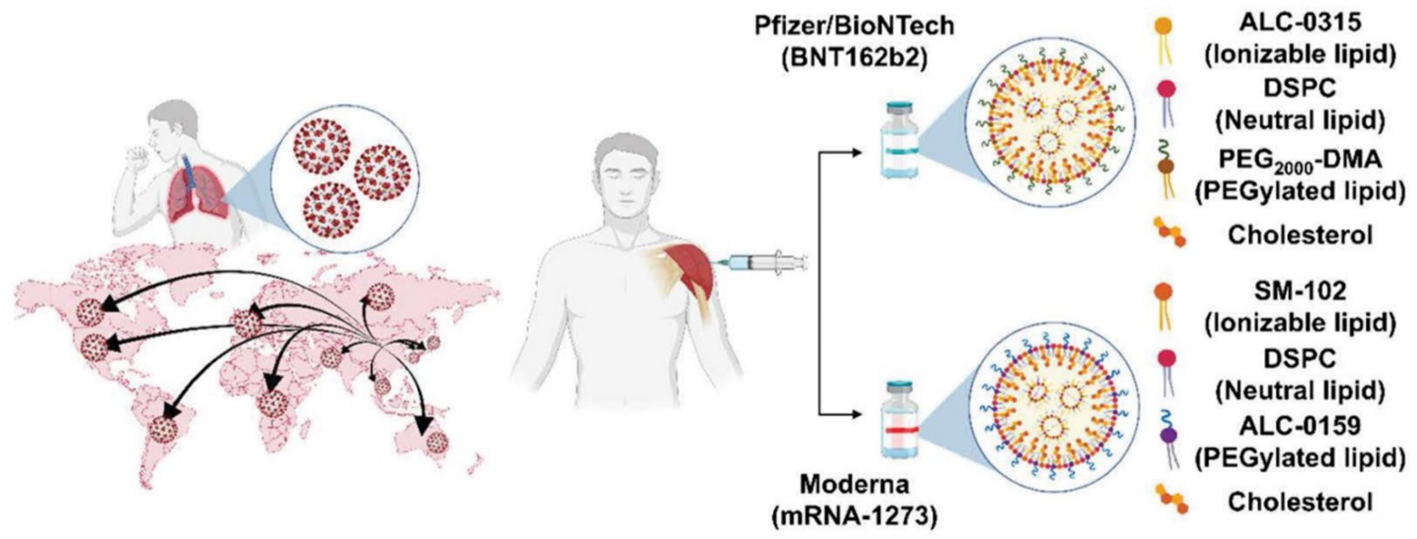
Figure 3. Development and representative lipid compositions of highly advanced mRNA vaccines used for combating COVID-19, specifically Pfizer/BioNTech’s BNT162b2 and Moderna’s mRNA-1273.
Based on the recent success of LNP-RNA based drugs, the development of more LNP-based RNA therapeutics is gaining momentum due to their potential in vaccines and therapeutics for various genetic diseases and cancers. One particular area of focus is the development of anticancer vaccines, which aim to induce an immune response against patient-specific neoantigens by utilizing lipid nanoparticles to deliver nucleic acids to specific organs or tissues.
References
[1] Rumiana Tenchov, Robert Bird, et al; ACS Nano 2021, 15, 16982-17015.
[2] Thai Thanh Hoang Thi , Estelle J. A. Suys, et al; Vaccines 2021, 9, 359.
[3] Han Na Jung, Seok-Yong Lee, et al; Theranostics 2022; 12, 7509-7531.